Prognosis for hematological malignancies treated with allogeneic stem cell transplantation (allo-HSCT) remains dismal and can often induce potentially deadly complications, such as graft versus host disease (GvHD). Steroids remain the first-line treatment for aGvHD. Nevertheless, new treatments are needed to improve allo-HSCT outcomes and reduce steroid complications. In this context, increasing evidences suggest that gut microbiota composition and the activity of regulatory T cells (Tregs) are involved in GvHD prevention (Elias and Rudensky, 2019, Br. J. Haematol. ; Shono and van den Brink, 2018, Nat. Rev. Cancer).
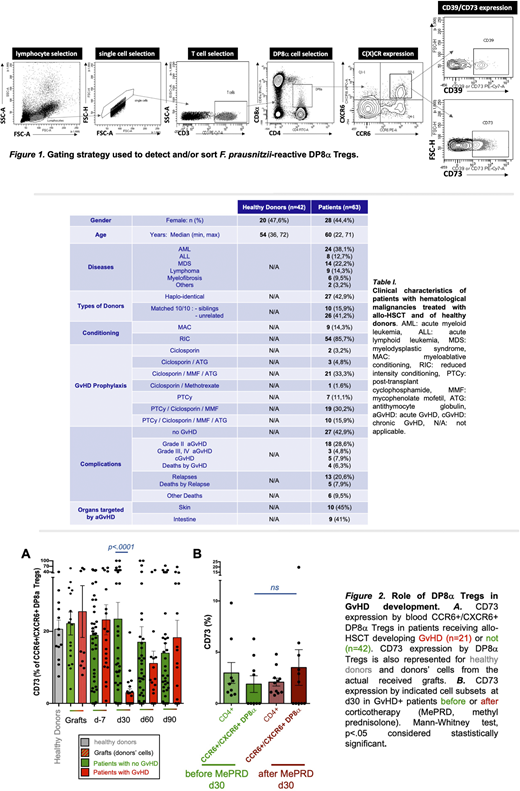
We have identified a novel FoxP3-negative IL-10-secreting Treg subset, named DP8α, enriched in the colon mucosa and present in blood, characterized by its CD3+/CD4+/CD8αLOW/ CCR6+/CXCR6+ phenotype and its TCR-specificity for the gut ClostridiumIV bacterium Faecalibacterium prausnitzii (Sarrabayrouse et al., 2014, PLoS Biol ; Godefroy et al., 2018, Gastroenterology). Sizable fractions of these cells also expressed the membrane-bound ectonucleotidases CD39 and CD73 (gating strategy shown in Figure 1), which are directly involved in their suppressive activity in vitro (Godefroy et al., 2018, Gastroenterology). In vivo, these molecules play key regulatory roles both by degrading extracellular ATP and thus limiting its inflammasome-related proinflammatory role, and by inducing the production of immunosuppressive adenosine. Accordingly, an alteration in adenosine/purinergic signaling has been suggested in GvHD occurrence (Deaglio et al., 2007, J. Exp. Med. ; Wang et al., 2013, PLoS ONE). Thus, we postulated that F. prausnitzii-reactive DP8α Tregs, whose suppressive activity depends on purinergic signaling, could bridge microbiota dysbiosis and GvHD incidence in allo-HSCT patients. In support of this, decreased levels of Clostridium bacteria, especially Faecalibacteriumspp, in patient's stools, have been associated with aGvHD risk (Noor et al., 2019, J Innate Immun). Moreover, mice gavaged with butyrate-producing and Treg-inducing Clostridia displayed milder GvHD and improved survival (Mathewson et al., 2016, Nat. Immunol.).
Therefore, we recently analyzed 63 patients with hematological malignancies, who received allo-HSCT, among which 21 developed an aGvHD (pathologies and treatments are listed Table I). We quantified circulating DP8α Tregs and analyzed their expression of CD39 and CD73 in donors, before and after mobilization, and in patients at one week before and 1, 2, and 3 months post allo-HSCT. Strikingly, results clearly showed that aGvHD development was strongly associated with a lack of expression of CD73 by DP8α Tregs (p<.0001) (Fig.2A), but not by any other T cell subsets analyzed. DP8α frequency and their CD39 expression were similar in GvHD+ versus GvHD- patients. Importantly, CD73 decrease did not result from their corticotherapy since it was equally observed before and after treatment (Fig.2B) and since in vitro treatment of PBMCs with methylprednisolone did not alter CD73 expression whatsoever. Therefore, the CD73 decrease observed in aGvHD patients, appears relevant and likely related to the aGvHD condition itself. It is noteworthy that this cohort was rather heterogeneous in terms of hematological diseases, conditioning regimens and prophylaxis treatments (Table I). Nevertheless, this drastic CD73 decrease in aGvHD patients was observed across all these conditions, strongly strengthening its relevancy.
Altogether, these data strongly suggest that a CD73-dependent functional alteration of DP8α Tregs are, at least in part, involved in aGvHD occurrence. These results could therefore be used to predict GvHD risk and also give rise to the development of therapeutic strategies. Such innovative treatments could be based on the infusion of DP8α Tregs with appropriate features, since we have shown both the uniquely high proliferating potential and the stable immunosuppressive function of these cells in vitro. Moreover, administration of DP8α target antigens (F. prausnitzii-derived), in the form of peptides, proteins, or even bacterial lysates, such as probiotics, could also be foreseen to either directly in vivo expand these Tregs or indirectly through the induction of tolerogenic dendritic cells (Alameddine et al., 2019, Front Immunol) and subsequent Treg differentiation/priming to limit GvHD-related inflammation.
Chevallier:Incyte Corporation: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal